This post is the last in a three-part series covering the management of beta-lactam allergies. Part 1 explained the enormous impact that penicillin allergies have on patient outcomes, while Part 2 discussed the different types of allergic reactions and the potential (or lack thereof) for beta-lactam allergy cross reactivity. This last post will cover the methods used to assess beta-lactam allergies. Let’s jump right in!
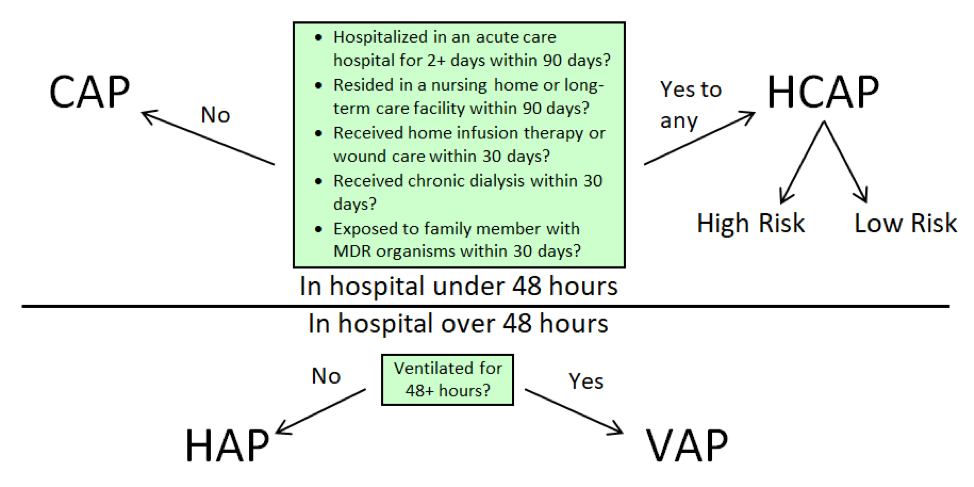
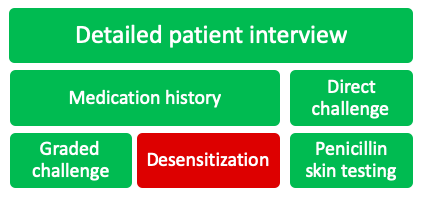
There are a variety of strategies that can be used to assess a patient’s beta-lactam allergy, each having their own place in the allergy assessment algorithm. The following will be detailed in this post:
Detailed Patient Interview
Far and away the most important step in assessing a patient’s beta-lactam allergy is a detailed patient interview. An allergy evaluation is recommended by many of the top health organizations in the country, including the Center for Disease Control and Prevention (CDC), National Quality Forum, Infectious Diseases Society of America (IDSA), American Board of Internal Medicine (ABIM), and the American Academy of Allergy, Asthma & Immunology (AAAAI).1 Just a minute or two of questioning the patient can yield an entirely different story than the allergy history in the medical chart. Some common questions I bring up with patients include:
- How many years ago did the reaction occur?
- What type of reaction did you have?
- Do you remember the details of the reaction? Did you have to go to the emergency room?
- How long after starting the medication did the reaction occur?
- How was the reaction managed?
- What happened when the medication was stopped?
- Have
you tolerated other forms of penicillin since the reaction? Have you had Keflex
(cephalexin), Augmentin (amoxicillin/clavulanate), or amoxicillin?
- Using brand names to question patients in this situation is important, as many patients wouldn’t recognize the jumble of letters that is amoxicillin/clavulanate
You can develop your own arsenal of questions to ask patients, but the important part is to talk to them. No further strategies are needed if you can rule out the documented allergy just from a 90-second conversation.
Medication History
The other piece that is absolutely necessary before proceeding is looking through the patient’s medication history yourself. If a patient with a documented penicillin allergy received ceftriaxone without issue on an admission last year, you can go ahead and give full-dose ceftriaxone during this admission if needed. The patient interview and medication history review can rule out >50% of documented allergies in my experience. In these situations, you can skip directly to the last section of this post: allergy re-labelling.
Direct Challenge
In patients with a very low probability of allergic reaction, a beta-lactam antibiotic can usually be given without pause. Situations where you can rule out an allergy based on patient interview or medication history can be “challenged” directly. This means giving the full dose of the preferred antibiotic and monitoring for any adverse effects. Some institutions also give a direct oral amoxicillin challenge with 250-500 mg of amoxicillin once prior to the intended beta-lactam initiation. If the patient can tolerate amoxicillin, any penicillin antibiotic can be given in the future without fear of experiencing an IgE-mediated reaction.
Graded Challenge
When you are not able to completely rule out an allergic reaction, a graded challenge is often the next logical step in hospitalized patients. Graded challenges are used when there is a low probability of an allergic reaction, but there is still a degree of discomfort giving the entire dose up front. In general, 10% of the full dose is given, the patient is monitored closely for 30 minutes, and then the full dose is given if no issues arise. If the patient tolerates these doses, you can rule out immediate hypersensitivity reactions and document the tolerance in the medical record, which will be discussed at the end of this post.
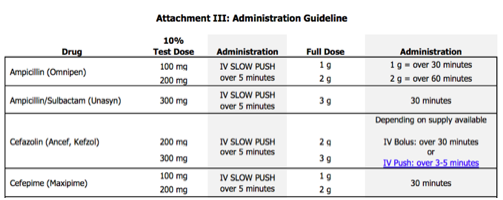
Desensitization
In patients who have confirmed or a high probability of severe IgE-mediated reactions to beta-lactams, but a beta-lactam is necessary for treatment, desensitization can be used. The desensitization procedure usually involves at least 12 doses of escalating concentrations of the required medication. This procedure requires incredibly close monitoring, which at most hospitals requires admission to the intensive care unit for administration. If a patient is able to tolerate desensitization, the patient must then begin regularly scheduled doses of the beta-lactam immediately upon the protocol completion. If doses are missed, the patient must be desensitized again. Desensitization does not rule out the allergy. The patient is still considered allergic to that agent, but can tolerate the medication for the course required in that instance.
Penicillin Skin Testing
Penicillin (PCN) skin testing has increased in popularity recently due to its relative ease of use and efficacy at ruling out IgE-mediated allergic reactions. In addition to rescue medications that should be handy just in case (diphenhydramine, methylprednisolone, and epinephrine) the skin test consists of 4 elements:
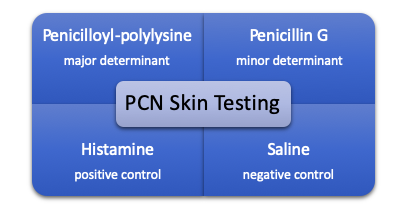
Initially, a percutaneous puncture test is done on the patient’s forearm with each of the elements and if tolerated, an intradermal test of each is also performed. The entire process generally takes around 45-60 minutes to complete and offers a negative predictive value for penicillin allergies of ~99%.2 Debate has surrounded the cost (both time and materials for the procedure), but multiple studies have now shown penicillin skin testing to be a cost-saving venture.2-5
Penicillin skin testing seems like a no-brainer, carrying the lowest risk of the procedures discussed thus far and its low overall cost for the health system. But in many institutions, it’s unclear who will perform the testing when allergy consultation is not available. In a 2015 survey of 736 infectious diseases providers, 57% responded saying that they do not have local options for skin testing.6 Does your institution?
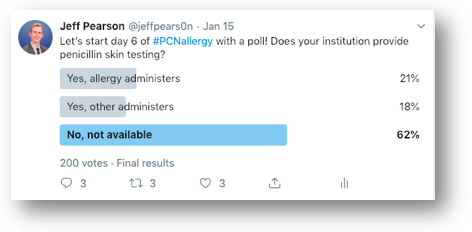
The people of Twitter have spoken and it resulted in similar responses, with 62% of respondents not having penicillin skin testing available at their institution. Previous studies have reported on the successes of penicillin skin testing performed by allergists,7-9 & many more antimicrobial stewardship programs,10 infectious diseases fellows/physicians,11 nurses,12 and pharmacists.13,14 If you’ve read this far into the post, you likely are interested in allergy skin testing, so I’d implore you to own the process if your institution doesn’t already have skin testing available! ALK provides some excellent instructional videos on their website to guide you through the testing process. Pharmacists aren’t licensed to perform skin testing in all 50 states, but they are in many of them, which this 2019 article did an admirable job exploring.15
Allergy re-labelling
The last fundamental step in navigating beta-lactam allergies is updating the patient’s allergy label. With all of the previous interventions, the allergy documentation can be further described in the medical record, with desensitization being the only intervention that does not rule out IgE-mediated reactions altogether.
In an ideal world, inaccurate allergy labels should be removed from the medical record. Unfortunately, this practice often leads to redocumentation of the allergy at a later admission however.16 Many hospitals have integrated innovative ways to improve this repetitive cycle, as seen via providers’ personal experiences here, here, and here. For those without the tech support for any of this functionality though, the best thing to do is to document, document, document.
Summary
The majority of penicillin allergy labels do not belong to patients with true allergies and these unnecessary labels lead to worse patient outcomes. We should all strive for more accurate and detailed allergy documentation in our patients, which all starts with a patient interview. All of the interventions discussed above can be used to remove/relabel a beta-lactam allergy, with the exception of desensitization.
For those looking to learn more, I highly recommend a recent review published in JAMA that goes into further detail on penicillin allergies.17 Make sure to check out the supplementary material too, it has some super helpful resources, including a full allergy toolkit for penicillin skin testing and oral amoxicillin challenges!
Previous posts in this series:
A Rash of Beta-Lactam Allergies, Part 1: The Problem
A Rash of Beta-Lactam Allergies, Part 2: The Education
References
- Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5:616-625
- Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health-Syst Pharm. 2017;74:232-7
- Mattingly TJ, Meninger S, Heil EL. Penicillin skin testing in methicillin-sensitive staphylococcus aureus bacteremia: A cost-effectiveness analysis. PLoS One. 2019; 14(1):e0210271. doi: 10.1371/journal.pone.0210271
- Jones BM, Avramovski N, Concepcion AM, Crosby J, Bland CM. Clinical and Economic Outcomes of Penicillin Skin Testing as an Antimicrobial Stewardship Initiative in a Community Health System. Open Forum Infect Dis. 2019;6(4): ofz109. doi: 10.1093/ofid/ofz109
- Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341-345
- Trubiano JA, Beekmann SE, Worth LJ, et al. Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the Emerging Infections Network. Open Forum Infect Dis. 2016; 3(3):ofw153
- Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140:154-161
- Macy E, Shu YH. The Effect of Penicillin Allergy Testing on Future Health Care Utilization: A Matched Cohort Study. J Allergy Clin Immunol Pract. 2017;5(3):705-710
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2006;97(5):681-687
- Leis JA, Palmay L, Ho G, et al. Point-of-Care β-Lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation. Clin Infect Dis. 2017;65(7):1059-1065
- Heil EL, Bork JT, Schmalzle SA, et al. Implementation of an Infectious Disease Fellow-Managed Penicillin Allergy Skin Testing Service. Open Forum Infect Dis. 2016;3(3):ofw155
- Macy E, Roppe LB, Schatz M. Routine Penicillin Skin Testing in Hospitalized Patients with a History of Penicillin Allergy. Perm J. 2004;8(3):20-24
- Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A Proactive Approach to Penicillin Allergy Testing in Hospitalized Patients. J Allergy Clin Immunol Pract. 2017;5(3):686-693
- Wall GC, Peters L, Leaders CB, Wille JA. Pharmacist-managed service providing penicillin allergy skin tests. Am J Health Syst Pharm. 2004;61(12):1271-1275
- Bland CM, Bookstaver PB, Griffith NC, et al. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am J Health Syst Pharm. 2019;76(3):136-147
- Rimawi RH, Shah KB, Cook PP. Risk of redocumenting penicillin allergy in a cohort of patients with negative penicillin skin tests. J Hosp Med. 2013;8(11):615-618
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188-199