The ATS and IDSA recently released the much-anticipated update to the community-acquired pneumonia (CAP) guidelines. The previous version was published back in 2007 and the new guidelines have included some major changes. Here is a rundown of all those changes that you need to know.
1. Health care associated pneumonia (HCAP) no longer exists
HCAP was an entity created with the 2007 CAP guidelines. It encompassed non-hospital acquired pneumonia in patients who had recent contact with the healthcare system. The recommendation was to treat HCAP with empiric broad-spectrum antibiotic therapy against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (PsA). With this strategy however, we were over-treating a lot of people. This study found that while 30% of all patients hospitalized for CAP received empiric anti-MRSA treatment, only 0.7% of all patients had MRSA pneumonia.
In the new guidelines, HCAP no longer exists. Instead, the guidelines emphasize assessment of risk factors for pathogens such as MRSA and PsA.

2. Treatment is now based on severity of the pneumonia rather than the location of the admitted patient
Prior guidelines differentiated antibiotic recommendations based on patient triage to the floor or the intensive care unit. In the new guidelines, treatment recommendations are based on the severity of the pneumonia, based on a list of criteria:

3. Only obtain blood cultures in severe CAP or if risk factors for MRSA and/or PsA are present
The new guidelines focus on cost-effective use of diagnostic tests.
Outpatient setting: recommend against any diagnostic testing (except for a chest X-ray)
Inpatient non-severe pneumonia: recommend blood cultures and sputum gram stain/culture ONLY if risk factors for MRSA and/or PsA are present
Inpatient severe pneumonia: recommend blood cultures, sputum gram stain/culture, Streptococcus pneumoniae urine antigen, and Legionella urine antigen and PCR/culture
*Legionella diagnostic tests are also recommended in times of an outbreak
These recommendations are based on literature demonstrating that:
- Overall prevalence of true positive blood cultures is 1-9% in patients with CAP3-6
- The majority of true positive blood cultures occur in patients with severe CAP6,7
- Blood culture results change clinical management in <2% of patients with CAP3,4,6
- The rate of blood culture contaminants is similar to the rate of true blood culture positives, resulting in unnecessary antibiotics and extended lengths of stay in the hospital3,4,6
4. Procalcitonin should NOT be used in the diagnosis of CAP
Procalcitonin is not a reliable marker for diagnosis of bacterial infections; it has roughly 65-75% sensitivity for detecting bacterial pneumonia8. Consequently, the risk of not treating bacterial CAP due to a low procalcitonin level can lead to poor outcomes. Although there is data to support use of procalcitonin in determining the duration of antibiotics in CAP9,10, the guidelines recommend use only in situations where duration exceeds the recommended 5-7 days.
5. The guidelines recommend use of the Pneumonia Severity Index (PSI) over the CURB-65 for determining need for admission
The argument from the guideline authors is that there is more literature in support for PSI in accurately predicting mortality rather than the CURB-65 score11-14. However, PSI incorporates data that may not be available in all circumstances, and certainly will not be available to the outpatient clinician who is trying to decide whether to admit a patient or not (such as pH, which can only be obtained from an arterial blood gas). So, although PSI may be recommended for use in the emergency department, the CURB-65 will likely remain in use, especially due to its efficiency in the outpatient setting.
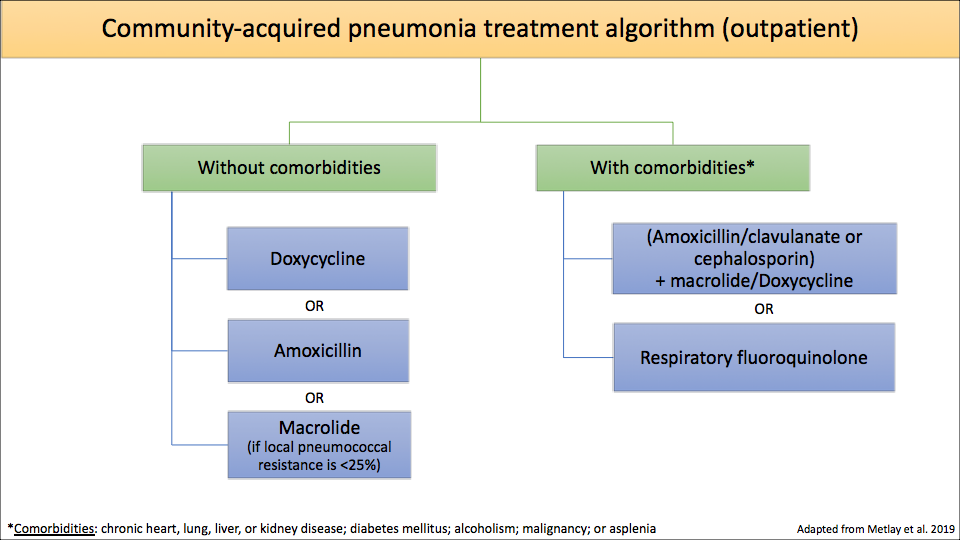
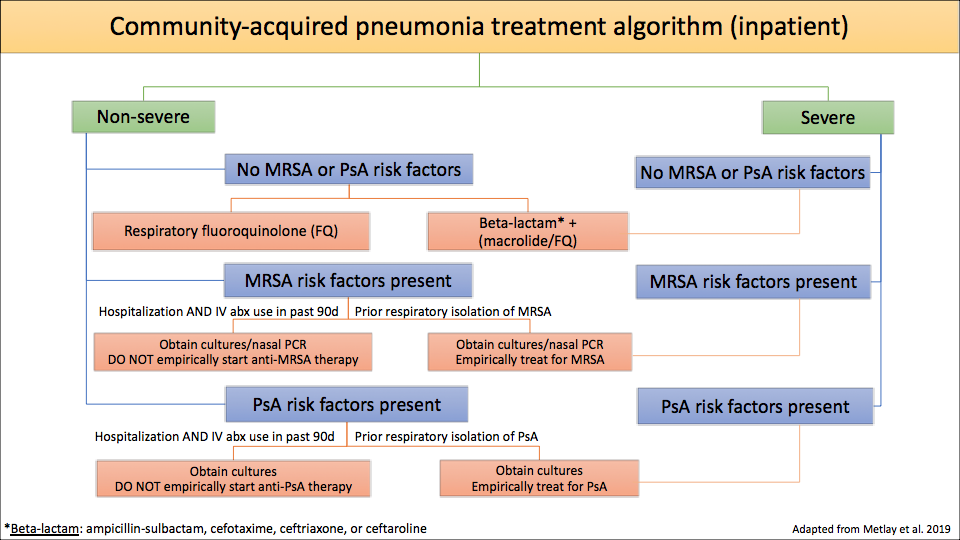
6. Algorithm for CAP antibiotic treatment
The meat of the guidelines is the treatment regimens – and there are quite a few changes.
- 1) Macrolides are no longer recommended as first line therapy in uncomplicated outpatient CAP unless the local streptococcal resistance to azithromycin is <25% (this study shows that most parts of the U.S. have resistance rates >25%).
- 2) Amoxicillin and doxycycline take the place of macrolides as first line treatment in uncomplicated outpatient CAP.
You may be thinking – “wait, amoxicillin doesn’t even cover atypical pathogens (i.e. Mycoplasma pneumoniae and Legionella pneumophila)!” This is true. But studies have shown that in otherwise-healthy patients, there was no difference in outcomes among those who received amoxicillin vs. an antibiotic that treats atypical organisms16. Exactly why that is remains unclear, but could be because healthy individuals clear the infection on their own or because the majority of these pneumonias are actually due to a virus, so they would improve with or without any antibiotics 5.
- 3) In hospitalized patients:
- Non-severe CAP – only treat empirically for MRSA and/or PsA if the organism has been isolated from the patient’s respiratory tract in the past
- Severe CAP – treat empirically for MRSA and/or PsA if the patient has any risk factors for MRSA and/or PsA respiratory infection
7. Treat anaerobes only in cases with suspected or proven lung abscess and/or empyema
Empiric treatment of anaerobes in aspiration pneumonia remains controversial, but the new guidelines recommend only treating anaerobes if there is suspicion for or a proven lung abscess and/or empyema.
8. Continue antibiotics for at least 48 hours in patients who are diagnosed with influenza pneumonia
This recommendation is based off the data that influenza infection predisposes to subsequent bacterial superinfections17 and a patient could have both a viral and a bacterial pneumonia at the same time. The guidelines state that if there is significant clinical improvement in 48 hours and no evidence to suggest a superimposed bacterial pneumonia, antibiotics can be discontinued at that time.
9. Duration of antibiotics is based on clinical improvement (but should be a minimum of 5 days)
Gone are the days of prespecified number of days for antibiotic duration. Instead, monitor the patient for signs of clinical improvement.
- If cultures are not growing MRSA and/or PsA, can stop empiric treatment for MRSA and/or PsA.
- If clinically improving, stop antibiotics following 48 hours of clinical improvement after a minimum of 5 days. Clinical improvement is determined by resolution of vital sign abnormalities, ability to eat/improved appetite, and normal mentation.
10. Do not use corticosteroids as adjunctive treatment and do not obtain routine follow up chest X-rays
These were not necessarily strategies that I employed prior to the publication of these guidelines, and corticosteroid use in CAP is controversial, but at this time, there is no strong data to support either of these adjunctive management strategies in patients with CAP.
References:
1. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Resp Crit Care Med. 2019; 200(7):e45-e67.
2. Self WH, Wunderink RG, Williams DJ, et al. Staphylococcus aureus Community-acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin Infect Dis. 2016; 63(3):300-309.
3. Chalasani NP, Valdecanas MA, Gopal AK, McGowan JE Jr, and Jurado RL. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995; 108(4):932-936.
4. Corbo J, Friedman B, Bijur P, and Gallagher EJ. Limited usefulness of initial blood cultures in community acquired pneumonia. Emerg Med J. 2004; 21(4):446-448.
5. Jain S, Self WH, Wunderink RG, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. New Eng J Med. 2015. 373:415-427.
6. Lee JH and Kim YH. Predictive factors of true bacteremia and the clinical utility of blood cultures as a prognostic tool in patients with community-onset pneumonia. Medicine (Baltimore). 2016; 95(41):e5058.
7. Waterer GW and Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med. 2001; 95(1):78-82.
8. Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized With Community-Acquired Pneumonia. Clin Infect Dis. 2017;65(2):183-190.
9. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017; 10:CD007498.
10. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107.
11. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005; 118(4):384-392.
12. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, and Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000; 283(6):749-755.
13. Carratala J, Fernandez-Sabe N, Ortega L, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142(3):165-172.
14. Renaud B, Coma E, Labarere J, et al. Routine use of the Pneumonia Severity Index for guiding the site-of-treatment decision of patients with pneumonia in the emergency department: a multicenter, prospective, observational, controlled cohort study. Clin Infect Dis. 2007;441(1):41-49.
15. Blondeau JM and Theriault N. Application of the Formula for Rational Antimicrobial Therapy (FRAT) to Community-Acquired Pneumonia. J Infect Dis Ther. 2017;5:313.
16. Postma DW, van Werkhoven CH, van Elden LJR, et al. Antibiotic Treatment Strategies for Community-acquired Pneumonia in Adults. New Eng J Med. 2015;372:1312-1323.
17. Metersky ML, Masterton RG, Lode H, File TM Jr, and Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16(5):e321-331.
1 thought on “Everything You Need To Know About the New ATS/IDSA Community-Acquired Pneumonia Guidelines”