This page will be updated weekly with new information on COVID-19
COVID-19 SUMMARY
You can also find this page via tabs at the top of the homepage
This page will be updated weekly with new information on COVID-19
COVID-19 SUMMARY
You can also find this page via tabs at the top of the homepage
There’s so much information about the novel coronavirus COVID-19 on the internet that it is hard to keep up with the onslaught of information. We wanted to compile the ultimate list of reputable resources for clinical providers to access when they need to, at a moment’s time.
Since information on COVID-19 is rapidly changing, these resources are not specific papers or blog posts, but rather websites that are maintaining up to date information on epidemiology, pathophysiology, and management. Resources span all types, including website behemoths like the WHO and CDC, as well as twitter accounts for people who get their news from social media.
Since this is an ultimate list but we don’t pretend to have ultimate knowledge of all resources, please send us resources that we may have missed and we will add them on here! We also acknowledge that this list is U.S. centric because we are from the States, but we would love input on resources for other countries so we can make this a more international list.
Graphics Reuters – for the graphic enthusiasts out there
New York Times Coronavirus Maps – for the graphic enthusiasts out there
That’s it for now! Let us know if we missed any great resources!
Thank you for all the work that all of you do. Stay safe!
This list was compiled by Milana Bogorodskaya, Fatima Al-Dhaheri, and Ahmed Abdul Azim.
Disease: Coccidioidomycosis
Alias: Valley fever, San Joaquin fever
Learning about fungi is hard enough even for infectious disease fellows (Narrator: especially for infectious disease fellows). By the time you learn how to differentiate the yeasts from the molds, the fungi kingdom decides to throw you a curve ball: Enter the shape shifters into the game of fungi learning – the dimorphic fungi.
The Dimorphic fungi shape shift depending on the weather (literally). They exist as molds in the great outdoors (environmental temperatures) and yeasts in the great indoors (inside our bodies at body temperatures). Clinically, this also means you will see the yeast forms in a histopathology review of a tissue sample, and our friends in the microbiology lab can re-create the environmental factors to grow them out as mold forms in culture. So essentially, they also shape shift between the microbiology lab and the pathology department. (They are sneaky Fung(uy)i…).
If you haven’t read the posts on Histoplasma capsulatum or Blastomyces species, go read it now!
This is the 3rd post out of 6 and will focus on our third shapeshifter, Coccidioides species.
CLICK HERE for a 2-page PDF handout of this information.
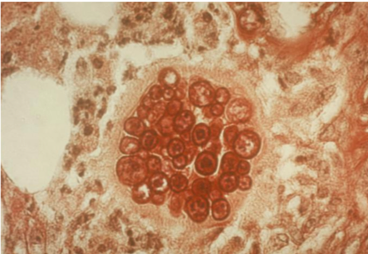
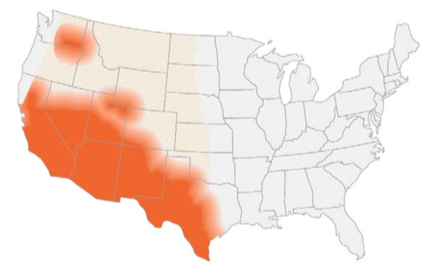
Your friendly Infectious disease doctors will always ask for tissue, and if classic spherules are seen in histopathology or culture confirms growth, that’s a slam dunk diagnosis! But we understand that is not always feasible, so in addition to clinical history + presentation, in order of importance:
Culture:
*Please alert the microbiology lab if you suspect coccidiomycosis and are sending them cultures! (culture needs to be specially handled in the lab due to the risk occupational transmission/infection — just like all dimorphic fungi covered in this review series).
Histopathology:
Antigen detection:
Serology:
Molecular methods:
Pulmonary disease:
Extra-pulmonary disease:
References:
Learning about fungi is hard enough even for infectious disease fellows (Narrator: especially for infectious disease fellows). By the time you learn how to differentiate the yeasts from the molds, the fungi kingdom decides to throw you a curve ball: Enter the shape shifters into the game of fungi learning – the dimorphic fungi.
The Dimorphic fungi shape shift depending on the weather (literally). They exist as molds in the great outdoors (environmental temperatures) and yeasts in the great indoors (inside our bodies at body temperatures). Clinically, this also means you will see the yeast forms in a histopathology review of a tissue sample, and our friends in the microbiology lab can re-create the environmental factors to grow them out as mold forms in culture. So essentially, they also shape shift between the microbiology lab and the pathology department. (They are sneaky Fung(uy)i…)
This is the first post out of 6 and will focus on our first shapeshifter, Histoplasma capsulatum.
CLICK HERE for a 2-page PDF handout of this information.
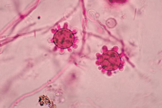
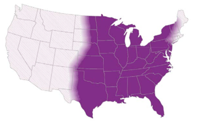
1. Culture:
2. Histopathology:
3. Antigen detection:
4. Serology:
5. Molecular methods:
| Clinical presentation | Mild/Moderate | Moderate/Severe | Chronic |
| Pulmonary | <4weeks: none >4weeks: itraconazole for 6-12 months | Lipid amphotericin B for 1-2 weeks followed by itraconazole for 12 weeks | Itraconazole for 12 months |
| Disseminated | Itraconazole for 12 months | Lipid amphotericin B for 1-2 weeks followed by itraconazole for 12 months | N/A |
References:
1. Climate change: the role of the infectious disease community. Lancet Infect Dis. 2017; 17:1219.
2. Greer A, Ng V, and Fisman D. Climate change and infectious diseases in North America: the road ahead. CMAJ. 2008; 178:715–722.
3. Walsh, TJ, Hayden, RT, and Larone, DH. Larone’s medically important fungi, 6th edition, ASM press, 2018.
4. Queiroz-Telles F, Fahal AH, Falci DR, et al. Neglected endemic mycoses. Lancet Infect Dis. 2017;17:e367–e377.
5. Azar MM and Hage CA. Laboratory Diagnostics for Histoplasmosis. J Clin Microbiol. 2017; 55:1612–1620.
6. Hage CA, Azar MM, Bahr N, Loyd J, and Wheat LJ. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015; 36:729–745.
7. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–132.
8. Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448–454.
9. Wheat LJ and Kauffman CA. Histoplasmosis. Infect Dis Clin North Am. 2003;17:1–19.
10. Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis. 2009; 49:1878–1882.
11. Saccente M and Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367–381.
12. Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825.